Unlock the potential of digital pathology for cancer diagnostics, research, and drug development with quantitative digital pathology. Improve productivity and quality by generating verifiable, reproducible, high quality analytical results in less time.
DISCOVERY
Tissue based biomarkers are essential to cancer drug development, patient stratification in conjunction with clinical trials, and for the development of companion diagnostics. Reveal answers to the toughest questions with ONCOtopix™ Discovery– a comprehensive solution for Cancer Research
- ONCOtopix Discovery is a comprehensive solution powered for whole slide image analysis of tissue sections, TMA slides across any image analysis application.
- Provides repeatable results with patented, automated and standardized solutions Visiopharm detection of tumor cells.
- Offers IHC analysis with standard analysis protocols for nuclei, membrane and cytoplasmic markers.
- Can operate unattended batch processing, search the database, automatically enter the results and identify the regions and cell structures. The results are combined interactively with allows you to display directly on the slide.
- Advanced tools allow you to calculate and plot sample statistic.
- ONCOtopix Discovery allows optimum use of available computing resources on a server or workstation, and thus gives the ability to group multiple slides in parallel.

VIRTUAL DOUBLE STAINING | VDS
Virtual Double Staining (VDS) is a novel and patent protected method for automated, robust and verifiable tumor and stroma separation in Tissue Microarrays and whole tissue sections.
- Significant intra- and inter reader variability is often associated with the diagnostic reading and interpretation of biomarker expression by pathologists. Virtual Double Staining has demonstrated significant improvements in both the reproducibility and accuracy of the assessment of biomarkers.
- VDS provides a robust and verifiable method to separate tumor versus stroma, thereby exclude proliferating lymphocytes which can be included when using global or hot spots during the assessment of biomarkers
- Virtual Double Staining uses TISSUEalign™ to adapt tumor markers for marker analysis. This provide a verifiable, automatic identification of cells cancer.
- VDS has been thoroughly tasted under practical conditions of clinical pathology in large laboratories in Euorope.
- Developed and validated in collaboration with NordiQC – independent scientific organization promoting the quality immunohistochemistry.
Benefits of Virtual Double Staining:
1. Fully automated Tumor/Stroma separation:
- Avoid time consuming manual outlining of tumor areas
- VDS performed by lab technicians
- Results confirmed or edited by the pathologist
- Can be integrated with LIS system
- Apply VDS to both full sections and TMA’s
2. Improve data quality
- Objective, verifiable detection of tumor
- Exclude proliferating lymphocytes
- Detect and exclude Ductal Carsinoma In Situ (DCIS)
3. Workflow that seamlessly applies to current laboratory operating procedures

TMAworkflow
Rapid and cost-effective screening of one or more biomarkers, across hundreds of tissue samples, is made easy and time efficient with TMAworkflow.
- A simple workflow wizard guides users step-by-step through the process of managing and working with TMAs.
- TMAWorkflow™ reads block designs, linking important donor ID with the position of a TMA core.
- Serial sections from the TMA block, stained with different biomarkers, are selected from the database. A work-list of all TMA slides is created, providing an overview of the entire study and all the slides and biomarkers that require analysis.
- With the click of a button TMAs are quickly de-arrayed, based on the block design overlay imported or created. Each core is maintained as a virtual slide even after de-arrayed. A small thumbnail is created in the database for each core, making navigation easy.
- Enable automated detection of tumor and co-localization studies with Virtual Double Staining and the multiplexing of biomarkers in TMAworkflow™.
- The entire test can be performed in batch mode without supervision. All results can be browsed, viewed and approved before the data are stored in a database where they will be attached to the core, providing full data integrity. The results are combined interactively with the positions of cores, which allows you to display directly on the slide.

ONCOtopix Dx
Unlock the potential of digital pathology for cancer diagnostics, research, and drug development with quantitative digital pathology. Improve productivity and quality by generating verifiable, reproducible, high quality analytical results in less time.

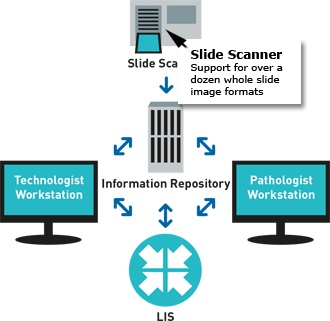
- Automated, robust, and verifiable quantitative immunohistochemistry (IHC) and gene probe analysis empowers technologists to handle all technical aspects of biomarker quantification. Results are pushed to the LIS system and presented to sub-specialized pathologists for review.
- Visiopharm’s patented, automated and standardized tumor cell detection and biomarker quantification has been proved to increase accuracy and reproducibility significantly.
- ONCOtopix Dx integrates with existing system AP-LIS of a laboratory-Access to the preparations for pathologists and technicians.
- All slides of patient, are automatically synchronized with him, ready to do simultaneous viewing and presenting the position of the pathologist. The results of analyzes are presented as an overlay on the images.
Media





